Sickle Cell Cure ‘Exa-cel’ To Receive Approval By December.
The Food and Drug Administration (FDA) is making significant strides toward approving a groundbreaking gene-editing drug that could offer new hope to individuals with sickle cell disease, a chronic inherited blood disorder that predominantly affects the Black community in the United States (Source: NBC News).
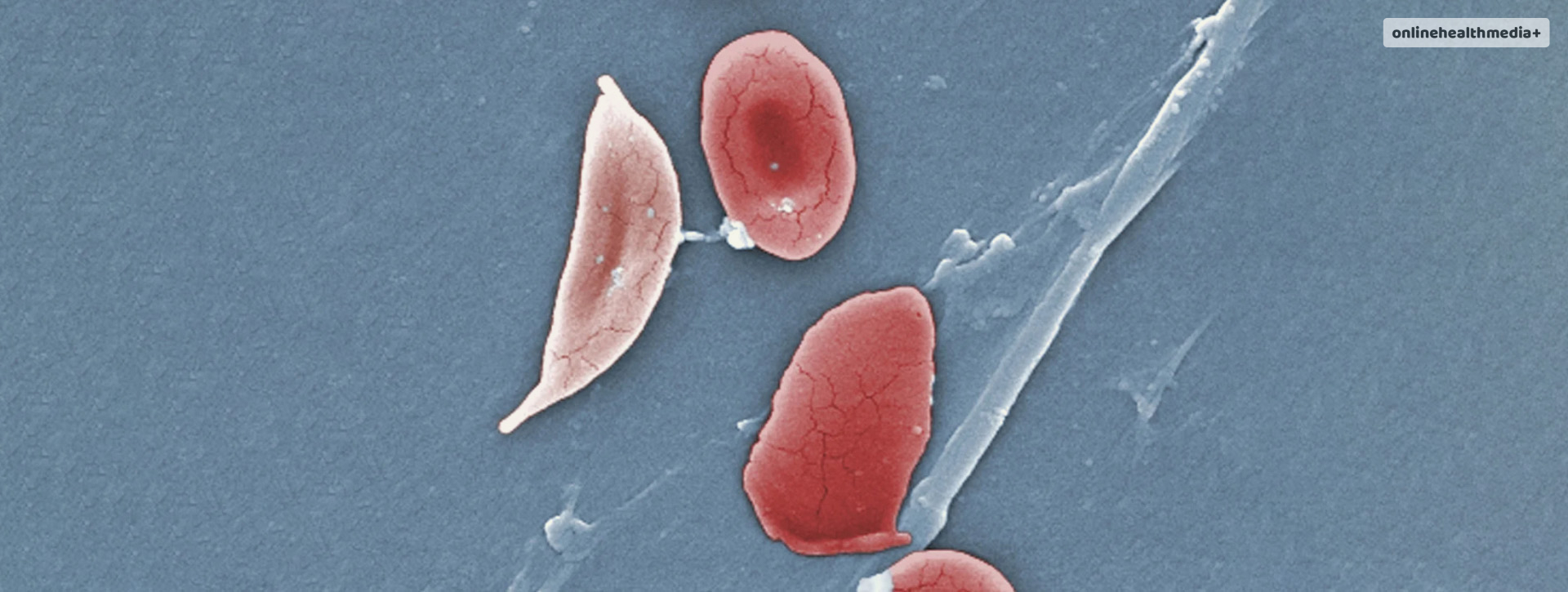
This innovative drug, named exa-cel, is developed to treat sickle cell disease, which currently affects around 100,000 individuals in the U.S. It’s a condition that transforms the usual disk-shaped red blood cells into a crescent or sickle shape.
Exa-cel represents a promising breakthrough that could replace the need for a bone marrow transplant, currently the only known cure for sickle cell disease but one that carries a risk of rejection.
This gene-editing drug, developed through a collaboration between Vertex Pharmaceuticals and CRISPR Therapeutics, works by modifying the patient’s blood cells’ DNA.
CRISPR, a groundbreaking gene-editing tool, targets specific DNA sequences, snipping out unwanted sections, including the genetic anomaly responsible for the crescent shape of blood cells in sickle cell disease. The treatment eliminates the need for an external donor and aims to permanently correct the patient’s own cells.
A recent FDA advisory committee meeting, which took place on Tuesday, signaled a crucial step toward exa-cel’s potential approval. During this meeting, the focus was primarily on assessing the “off-target” effects of CRISPR.
These effects occur when the gene-editing tool makes unintended cuts in DNA. The outcome of such edits depends on where they happen in the patient’s genetic code and remains uncertain.
Vertex Pharmaceuticals presented data from a study involving 46 patients who received exa-cel treatment. Among the 30 patients with at least 18 months of follow-up, 29 experienced no severe pain crises.
The company claimed there was no evidence of off-target effects. However, the committee expressed a desire for more comprehensive data before making a final decision.
If approved, exa-cel is expected to be a game-changer for sickle cell disease patients. Nevertheless, the price tag for gene therapy is anticipated to be significant, possibly reaching as high as $2 million per patient, according to the Institute for Clinical and Economic Review. Ensuring patient access will be a critical consideration.
The FDA is set to announce its final decision on exa-cel by December 8, 2023. If approved, this groundbreaking gene-editing drug could offer a potential cure for sickle cell disease, offering new hope and improved quality of life to thousands of affected individuals.
Also Read
- Top Automated Wheelchairs Of 2023
- Different Types Of Dementia: A Comprehensive Overview.
- 10 Things You Should Know About Having Dental Retainers.